A Queueing Approach to Reduce Phase I Study Duration in Oncology
Try the queueing study simulations with the FlexSimHC™.
A Queueing Approach to Reduce Phase I Study Duration in Oncology
Try the queueing study simulations with the FlexSimHC™.

Read the full paper published on Statistics and Research Methods
Paul H. Frankel1, Vincent Chung2, Joseph Tuscano3, Tanya Siddiqi4, Jeffrey Longmate1, Susan Groshen5, Edward M. Newman2,6
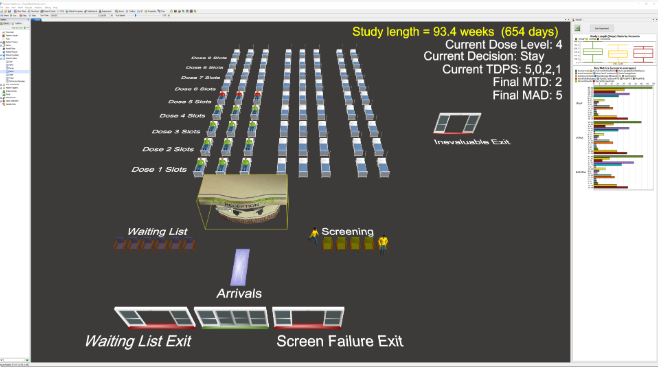
We seek to reduce the duration of Phase I studies in adult and pediatric cancer studies without violating risk-limits by better accommodating the accrual and evaluation process (“queue”). The processes modeled, the Phase I Queue (IQ), includes patient inter-arrival time, screening, and dose-limiting toxicity evaluation. For our proof-of-principle, the rules of the 3+3 and Rolling 6 Phase I designs were modified to improve patient flow through the queue without exceeding the maximum risk permitted in the parent design. The resulting designs, the IQ 3+3 and IQ Rolling 6, were compared to their parent design by simulation in twelve different scenarios. We demonstrate that IQ designs can reduce the average duration of Phase I studies as compared to their parent designs without changing the risk-limits or MTD selection operating characteristics. These approaches have been successfully implemented in both hematology and solid tumor Phase I studies.
1Division of Biostatistics, City of Hope, 2Department of Medical Oncology, City of Hope, 3Hematology/Oncology, University of California at Davis, 4Hematology and Hematopoietic Cell Transplantation, City of Hope, 5Biostatistics Core, University of Southern California/Norris Cancer Center, 6Division of Molecular Pharmacology/Developmental Cancer Therapeutics Program, City of Hope, Duarte, CA, USA.